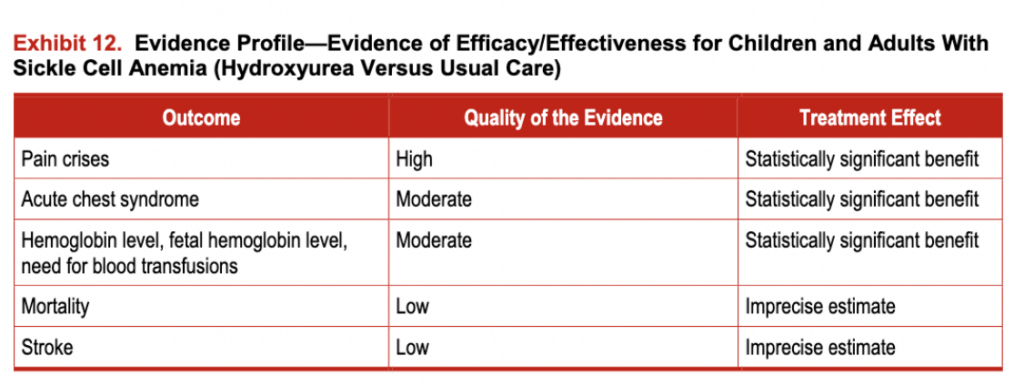
A comprehensive systematic review was conducted evaluating the efficacy, effectiveness, harms, and barriers associated with using hydroxyurea in SCD. Three randomized trials and 54 observational studies describing the use ofhydroxyurea in adults (n=21) and children (n=33) were identified. Exhibit 11 describes participant characteristics for the randomized trials, and exhibit 12 includes the evidence profile of efficacy/effectiveness for hydroxyurea in patients with SCA.
Evidence of Efficacy/Effectiveness
Summary of Evidence in Adults With SCA
The Multicenter Study of Hydroxyurea in Patients With Sickle Cell Anemia (MSH) was a randomized, double blind, placebo-controlled trial involving 299 adults with SCA who had experienced three or more VOCs in the previous year. The clinical end point of three or more documented VOCs was chosen because of earlier data documenting that people who experience pain at that frequency had markedly lower survival rates. The MSH trial demonstrated that, compared to placebo, hydroxyurea therapy reduced the frequency of painful episodes and ACS events, as well as the need for RBC transfusions and hospitalizations. In 1998, based on the results of this trial, the U.S. Food and Drug Administration approved hydroxyurea for the treatment of clinically severe SCA in adults.
Summary of MSH Findings
Lower annual rates of pain crises (median 2.5 crises per year vs. 4.5 crises per year)
Longer time to a first crisis on study (3.0 months vs. 1.5 months) and longer time to a second crisis (8.8 months vs. 4.6 months)
Lower incidence of ACS (25 patients vs. 51 patients)
Reduced need for blood transfusions (48 patients vs. 73 patients)
Increased total hemoglobin (0.6 g/dL) and HbF (from 5.0 to 8.6 percent in the intervention group), compared with a drop in the placebo group (from 5.2 to 4.7 percent)
Lower costs for hospitalization for pain ($12,160 in the hydroxyurea group versus $17,290 in the placebo group)
Differences in the effect on mortality and stroke outcomes were not statistically significant.
Over 2 years of treatment, the benefit of hydroxyurea on quality of life was limited to people who maintained increased HbF levels. These restricted benefits occurred in social function, pain recall, and general health perception. Annualized total costs were similar between the intervention group and the placebo group. The trial had low risk of bias but was stopped early for benefit, which may exaggerate the observed benefit. Supporting evidence from 21 observational studies involving 3,378 adults, with followup periods of 24-96 months, was consistent in showing a reduction in pain crises (60-90 percent), hospitalizations (90-100 percent), and an increase in HbF (4-20 percent).
A 9-year followup analysis ofMSH participants indicated a reduction in mortality for the group of people who took hydroxyurea compared to those who did not take the medication.374 When the cohort was followed for up to 9 years, people taking hydroxyurea had 40 percent reduced mortality (analysis according to cumulative hydroxyurea exposure, not the original randomization). Survival was related to HbF levels and frequency of vaso-occlusive crises. More recently, extension of the followup analysis to 17.5 years for nonrandomized people indicated continued safety and benefit of hydroxyurea, including reduced mortality. Results were published from another prospective clinical study of hydroxyurea therapy with 17-year followup analysis. This prospective, nonrandomized study was conducted in Greece and enrolled people older than 16 years who had HbSS or HbSp0-thalassemia, and HbSp+-thalassemia. Similar to the results of the MSH trial, the results from this study showed that hydroxyurea therapy reduced the frequency of painful episodes and ACS events and the need for RBC transfusions and hospitalizations. Hydroxyurea therapy also significantly improved survival when compared to conventional therapy.
With randomized trials, both stopping the trial early and imprecision (single trial with <300 events) can affect the quality of the evidence. However, the overall quality of the evidence is considered high because the supporting observational evidence and the large treatment effect that follows hydroxyurea administration strengthen inference. Summary of Evidence in Children With SCA For infants, children, and adolescents who have SCA, hydroxyurea treatment results have closely and consistently mirrored those of adults. The first large, prospective, multicenter phase I/II trial (HUG KIDS) of school-aged children who were treated with hydroxyurea escalated to the maximum tolerated dose demonstrated laboratory efficacy, few short-term toxicities, and lack of toxicity for childhood growth and development. Soon after, a prospective phase I/II trial of infants with SCA who were treated with a liquid hydroxyurea formulation at a fixed dose of 20 mg/kg/day generated favorable short-term safety data and evidence suggesting prevention of sickle cell-related organ damage.
Subsequently, several groups in the United States and Europe published open-label data regarding the laboratory and clinical efficacy ofhydroxyurea for young people with SCA, with evidence of sustained laboratory and clinical responses but without apparent long-term toxicities. Taken together, these trials provide almost 15 years of pediatric data on both the safety and efficacy of hydroxyurea for young people (reviewed in Ware 2010).372 Most recently, the phase III double-blinded, placebo-controlled infant hydroxyurea study “Pediatric Hydroxyurea phase III Clinical Trial had equivocal results for preservation of organ function, but confirmed the improvements in laboratory parameters such as total hemoglobin level and HbF level, and decreased numbers of sickle-related acute clinical events such as pain and ACS. Long-term observational studies suggest sustained beneficial effects of hydroxyurea for young people without excessive myelotoxicity, deleterious effects on growth and development, altered fertility, accumulation of mutations, or increased carcinogenicity.
Exhibit 11. Participant Enrollment Criteria for Placebo-Controlled Randomized Controlled Trials of
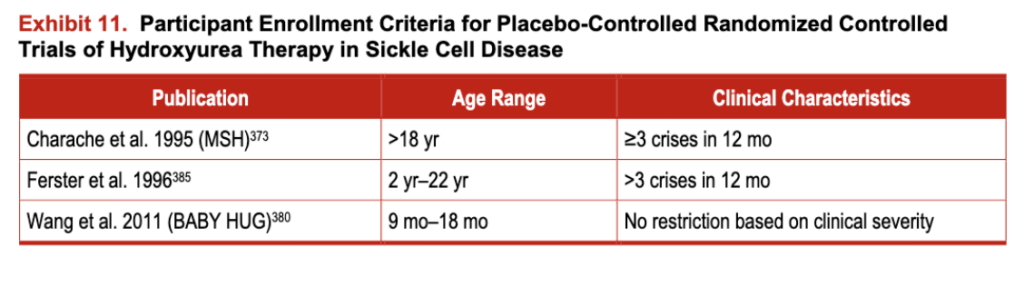
Exhibit 12. Evidence Profile-Evidence of Efficacy/Effectiveness for Children and Adults With Sickle Cell Anemia (Hydroxyurea Versus Usual Care)
Summary of Evidence in People With Genotypes Other Than HbSS or HbSj3°-Thalassemia
There have been no phase III trials ofhydroxyurea therapy in people with SCD having genotypes other than HbSS or HbSp0-thalassemia. The prospective, nonrandomized study from a major clinical center in Greece referred to earlier enrolled 165 people with HbSp+-thalassemia. Only 44 people were receiving hydroxyurea. Data analysis was based on all subjects enrolled in the study, with the majority receiving hydroxyurea therapy represented by people with SCA. The overall 10-year survival for the subset of people with HbSp+-thalassemia receiving hydroxyurea was not significantly different from those receiving conventional therapy. A phase II study of children and adults with HbSC evaluated the effects of hydroxyurea and magnesium pidolate on laboratory parameters. The study was closed due to slow enrollment, and only 36 people were evaluable for the primary outcome of proportion of hyperdense cells after 8 weeks; no difference was seen for people receiving hydroxyurea. In addition, people receiving hydroxyurea had favorable hematological effects with increased HbF and RBC MCV, which were not observed for people receiving only magnesium pidolate.
Evidence of Side Effects
The evidence for hydroxyurea toxicity in people with SCD is derived from three RCTs that enrolled 517 people and from 47 observational studies that enrolled more than 3,000 people. In people who do not have SCD, toxicity evidence is derived from 21 RCTs that enrolled more than 4,800 individuals and 35 observational studies that enrolled more than 7,500 individuals (see exhibit 13). (For more information, see the evidence table at http://www.nhlbi.nih.gov/guidelines/scd/index.htm).
Exhibit 13. Evidence Profile-Evidence of Side Effects in Sickle Cell Anemia
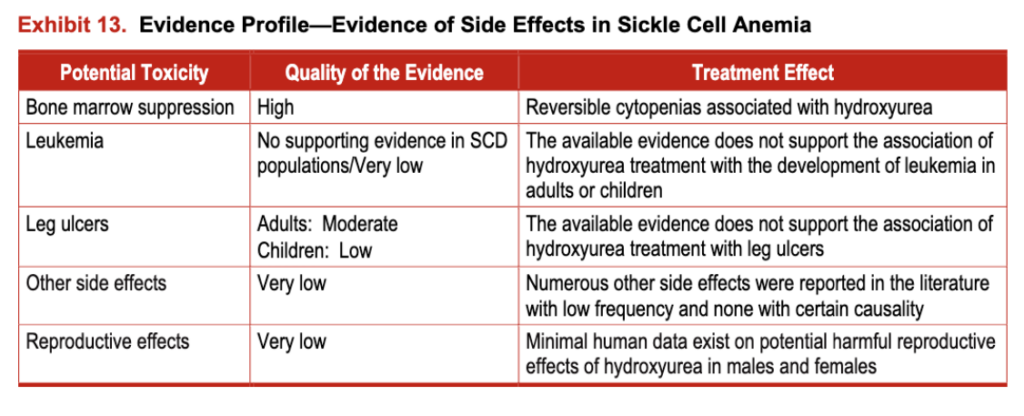
Minimal human data exist on potential harmful reproductive effects of hydroxyurea in males and females
Evidence Supporting Use of a Treatment Protocol
Although the literature does not compare different implementation protocols for hydroxyurea, the expert panel was concerned that not using a protocol could lead to inadequate dosing or poor monitoring. Hence, to maximize the benefits and safety ofhydroxyurea treatment, the expert panel strongly recommends adopting a standardized protocol based on the available evidence. The expert panel developed a suggested protocol based on (1) protocols used in published clinical trials and observational studies, (2) indirect evidence derived from basic science and pharmacokinetics ofhydroxyurea, and (3) a consensus process. Although the protocol contains several technical remarks and recommendations needed to implement hydroxyurea therapy safely and effectively, it should be considered as guidance and modified to fit an individual patient’s clinical situation (see “Consensus Treatment Protocol and Technical Remarks for the Implementation ofHydroxyurea Therapy”).
Additional Considerations
Guideline developers consider what is known in the literature about people’s values and preferences and make assumptions about values demonstrated by people encountered in clinical practice. In the area of SCD, the evidence supporting the nature and distribution of people’s values is not strong. However, the expert panel has considered patients’ values in their decision making process. In one study of pediatric patients and their caregivers, parents and children indicated a preference for hydroxyurea over other therapies such as routine RBC transfusions or stem cell transplantation. The benefit/harm balance seems to be the driving determinant of treatment choice in this study. In developing these recommendations, the expert panel placed high value on preventing SCD morbidity (specifically, VOCs and ACS) and low value on cost, burden, and the potentially unknown long-term adverse effects of hydroxyurea therapy.
Although the hydroxyurea clinical trials cited in this chapter used very restrictive definitions for chronic, acute, and recurrent pain, the panel has chosen to broaden the definitions by using information from people in observational studies and clinical trials. For example, for this document, the expert panel defined recurrent SCD-associated pain to include daily pain requiring the use of opioid medication. In addition, the expert panel also includes those people who have episodes of pain, which, in the view of the patient and provider, significantly affect activities of daily living and quality of life.
It is the nature of most efficacy clinical trials to restrict enrollment to people with substantial clinical severity. Unfortunately, this limits data on the majority of people who do not fit easily into the restrictive clinical trial definitions-that is, the people who are seen in everyday practice. Notably, the pediatric phase III trial (BABY HUG) did not have specific inclusion criteria based on clinical severity; even infants with no previous clinical VOCs were eligible for enrollment. This was intentional and designed to allow the findings to be generalized to all infants and toddlers with SCA.
The panel deliberated extensively on using data from clinical trials alone, which in most cases would limit the use of hydroxyurea to people who have had three or more pain crises in the last year. However, the panel felt that this would prevent the use of hydroxyurea in some adults who have chronic, acute, and recurrent pain, and for whom observational studies have generally shown a benefit from the medication. Therefore, in an effort to include the broad range of pain syndromes that affect the ability of people with SCD to participate in their desired daily activities, the panel’s definitions of chronic, acute, and recurrent pain and their recommendations for the use of hydroxyurea have been expanded beyond the eligibility criteria used in the clinical trials. Thus, the expert panel believes that the use of hydroxyurea is indicated in a broader range of individuals than those described in the inclusion criteria for MSH and hopes to encourage use of the medication in people who have acute and/or chronic pain that regularly interferes with their quality of life.
In addition, when issuing recommendations for adults, the expert panel occasionally used data from the pediatric SCD literature and data from populations without SCD who were treated with hydroxyurea. In particular, this occurred in the areas of evidence of harm and treatment initiation and monitoring. The panel acknowledges that this indirect evidence is of lower quality and associated with weaker inferences.
